When atoms or molecules in a solid are put under extremes of pressure, temperature or magnetic fields, extraordinary things can happen. Not only can the crystalline structure change from one state to another, but so can the atoms themselves! For example, if an element such as Rubidium is subjected to high enough pressure it converts itself spontaneously into a completely new kind of material: a self-alloy where two different sizes of Rubidium atom (with different electronic states) occupy different positions in a "guest-host" structure.
Within SUPA, staff at Edinburgh's multidisciplinary Centre for Science at Extreme Conditions (CSEC) has discovered many examples of new atomic and molecular materials, by applying pressures previously encountered only at the heart of giant planets such as Jupiter. One major ambition is to recover some of these new materials to ordinary conditions without losing the new structure and properties. Nature has set an outstanding example of exactly this process: the formation of diamonds.
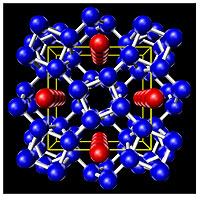 |
The structure of Rubidium at 18GPa (180,000 Atmospheres). Red and blue atoms are both Rubidium but have different sizes for the guests (red) and the hosts (blue). This structure is nicknamed the "Rubidium Hotel". (Image: M. McMahon, Edinburgh.) |